Cell free DNA in maternal blood
Clinical implementation
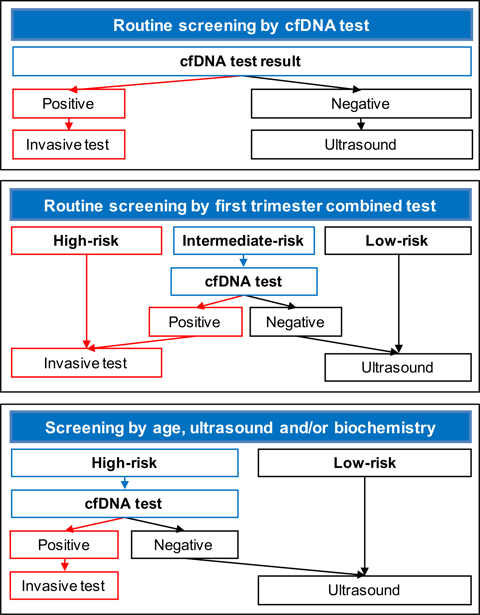
There are essentially two options in the clinical implementation of the cfDNA test in screening for trisomies 21, 18 and 13:
- Routine screening of the whole population. The test could be offered from as early as 10 weeks’ gestation; at earlier gestations there is a high risk of test failure. The limiting factor for this approach is the relatively high cost of the test which varies between 300 and 1000 €.
- Offer the cfDNA test to a subgroup of the population selected after first-line screening by another method, preferably the first-trimester combined test. Such approach would lead to very high detection rate and very low invasive testing rate at a considerably lower cost than carrying out the cfDNA test in the whole population.
- The selected subgroup for cfDNA testing could be either the one at high-risk or the one at intermediate-risk.
In any case all women should also be offered a detailed scan at 11-13 weeks for early detection of major defects and early screening for preeclampsia to identify a high-risk group that would benefit from treatment with aspirin.