Screening for preeclampsia
Overview
Preeclampsia (PE), which affects about 2% of pregnancies, is a major cause of perinatal and maternal morbidity and mortality.
- It is preterm PE, requiring delivery before 37 weeks, rather than term PE which is associated with an increased risk of perinatal mortality and morbidity and both short-term and long-term maternal complications.
The underlying mechanism for preterm PE is thought to be impaired placentation, documented by the findings of abnormal blood flow in the uterine arteries and reduced maternal serum levels of placental products.
The patient-specific risk of developing PE can be predicted by a combination of factors in the maternal history, including Black racial origin, high body mass index and prior or family history of PE, and the following measurements taken at 11-13 weeks:
- maternal blood pressure
- uterine artery pulsatility index (PI)
- maternal serum level of PLGF
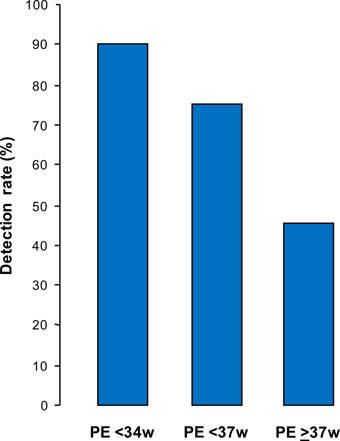
Screening by this combined approach could identify about 90% of patients that develop PE requiring delivery before 34 weeks, 75% of those with preterm PE and 45% of term PE, at a false positive rate of 10%.
A randomized study (ASPRE trial) demonstrated that in singleton pregnancies identified by first-trimester screening as being at high-risk of preterm PE (>1 in 100), administration of aspirin (150 mg/day) from 12 until 36 weeks' gestation reduced the incidence of PE before 34 weeks by >80% and PE before 37 weeks by >60%.