Performance of screening
Screening at 11-13 weeks
The objective of screening for PE at 11-13 weeks’ gestation is to identify the cases that would benefit from prophylactic use of aspirin that reduces the risk of preterm PE by more than 60%.
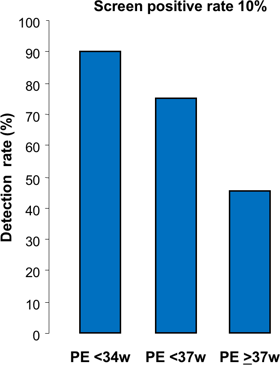
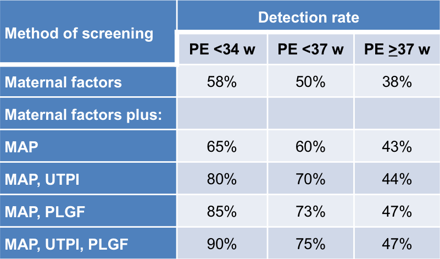
Combined screening by maternal factors, MAP, UTPI and PLGF predicts about 90% of early PE (<34 weeks), 75% of preterm PE (<37 weeks) and 45% of term PE (≥37 weeks), at screen positive rate of 10%.
- Inclusion of PAPP-A and sFLT-1 does not improve the performance of screening.
The traditional approach of identifying women at high-risk of PE that could benefit from aspirin is based on maternal factors. The National Institute for Health and Clinical Excellence (NICE) in the UK recommends the identification of the high-risk group on the basis of 10 factors from maternal characteristics and medical history; this method identifies only about 40% of cases of preterm PE and 35% of term PE. The American College of Obstetricians and Gynecologists (ACOG) recommends the use of aspirin in women with a history of PE in ≥2 pregnancies or history of PE with delivery <34 weeks; this method identifies 5% of cases of preterm PE and 2% of term PE.
- O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Am J Obstet Gynecol 2016;214:103.e1-103.e12.
- National Collaborating Centre for Women’s and Children’s Health (UK). Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. London: RCOG Press, 2010.
- Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122: 1122-31.
- O’ Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, Carbone IF, Dutemeyer V, Fiolna M, Frick A, Karagiotis N, Mastrodima S, de Paco Matallana C, Papaioannou G, Pazos A, Plasencia W, Nicolaides KH. Multicenter screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation: comparison to NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol 2017; 49: 756-60.