Background to preeclampsia
Pathogenesis
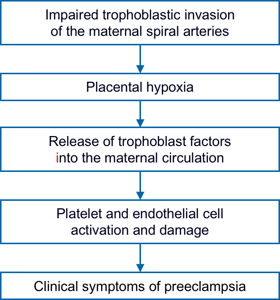
Reduced perfusion of the placenta causes oxidative stress which in turn triggers off release of trophoblast-derived factors which enter the maternal circulation and cause endothelial cell damage in the kidney, liver, brain and placenta and an exaggerated inflammatory response which underlines many of the changes observed in PE.
Placental-derived factors released in response to stress include the anti-angiogenic protein sFLT1, which is increased in PE, whereas the circulating concentration of the angiogenic placental growth factot (PlGF) is reduced in PE. This angiogenic imbalance results in increased maternal vascular inflammation and generalized endothelial dysfunction.
In contrast to preterm PE, which is characterised by impaired placentation, in term PE placentation is usually normal. In women with medical disorders, such as chronic hypertension, there is endothelial dysfunction even before pregnancy. In such cases PE can develop in the absence or lower degree of impaired placentation; the pre-existing endothelial dysfunction is further exacerbated by the physiological burden of pregnancy, as normal pregnancies carry a low-grade systemic inflammatory response.